Abstract
Multiple myeloma is a clonal proliferation of malignant plasma cells and in the vast majority of patients remains incurable. The biology of the malignant plasma cells (PCs) in multiple myeloma (MM) is highly influenced by the bone marrow (BM) microenvironment in which they reside. Specifically, BM stromal cells (SCs) have been shown to interact with MM cells to promote their growth, survival and resistance to conventional chemotherapy. In contrast, it remains unclear how the BM microenvironment modulates the response of MM cells to novel agents, such as proteasome inhibitors or responses to innate immune cells. Moreover, it remains unclear which specific cell types within the BM modulate the therapeutic response of MM cells.
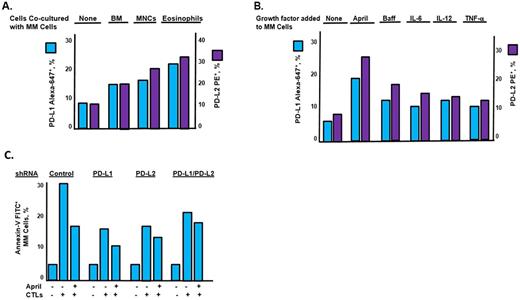
MM cell lines and patient tumor cells co-cultured with BM and BM-derived eosinophils demonstrated increased growth, proliferation and survival in the presence of the proteasome inhibitors bortezomib and carfilzomib. BM eosinophils have been reported to support the survival of plasma cells by secreting APRIL (a pr oliferation- i nducing l igand), among other growth factors, and contribute to the long-term maintenance of malignant plasma cells. Results indicated that addition of APRIL to MM cultures increased cell proliferation.
Targeting immune checkpoints such as programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and PD-L2 has achieved noteworthy benefit in multiple cancers by blocking immuno-inhibitory signals and enabling patients to produce an effective antitumor response. Programmed death-ligand 1 (PD-L1) and PD-L2 are ligands for the receptor PD-1 within a negative costimulatory network that leads to a T cell exhaustion phenotype, preventing appropriate cellular response to antigens. Treatment with patient BM, BM-derived eosinophils or APRIL led to a substantial upregulation of PD-L1 and PD-L2 on the surface of MM cells (Fig. 1A, B). Furthermore, pre-treatment of MM cells with eosinophils or APRIL suppressed anti-tumor immunity by cytotoxic T lymphocytes (CTLs). shRNA-mediated knockdown of PD-L1 and PD-L2 on MM cells enhanced the cytotoxic effect of CTLs (Fig. 1C). Treatment with the FDA-approved proteasome inhibitors bortezomib, carfilzomib and ixazomib also dramatically and rapidly upregulated PD-L1 and PD-l2 levels on MM cells. Treatment of MM cells with proteasome inhibitors also compromised the anti-myeloma effect of CD8+ T cells. Chemical compound screening was then performed and revealed that small molecule kinase inhibitors that suppressed the basal and drug-induced expression of PD-L1 and PD-L2 on MM cells. Novel small molecule kinase inhibitors that suppressed PD-L1 and PD-L2 on MM cells also triggered CTL activation with antimyeloma benefit.
Conclusions: BM-derived eosinophils enhanced immuno-suppressive microenvironmental effects and promoted resistance of MM cells to all proteasome inhibitors tested. APRIL, secreted by eosinophils, promoted MM growth and upregulated the checkpoint proteins PD-L1 and PD-L2 on MM cells. Thus, standard-of-care antimyeloma agents such as proteasome inhibitors as well as the BM-secreted cytokine APRIL suppressed antimyeloma immunity. Antibody-mediated blockade of the PD-L1/PD-L2~PD-1 axis restores antitumor immunity in cancer patients with therapeutic benefit, but exhibits numerous adverse side effects. The development of novel small molecules that suppress checkpoint proteins on MM cells may overcome the drawbacks associated with monoclonal-based checkpoint inhibitors to provide greater specificity, increased tumor response rates and fine-tune therapy while managing adverse events. Small molecules also provide increased oral bioavailability and higher stability at ambient temperature facilitating purification during clinical production. Kinase inhibitors that suppress PD-L1 and PD-L2 to promote antimyeloma immune effects may also limit tumor progression in immunocompetent murine models of myeloma.
Malek: Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal